Uganda moves forward on PrEP vaginal ring and injection
Hester Phillips
20 December 2022
More choice for HIV prevention could be on the horizon in Uganda as the government approves a PrEP vaginal ring and is close to approving injectable PrEP
Uganda is one of the first African countries to approve a PrEP vaginal ring and is close to approving injectable PrEP.
What is this story about?
In Uganda, two new PrEP products for HIV prevention are approved or close to approval: the dapivirine vaginal ring and the long-acting injectable cabotegravir (CAB-LA)
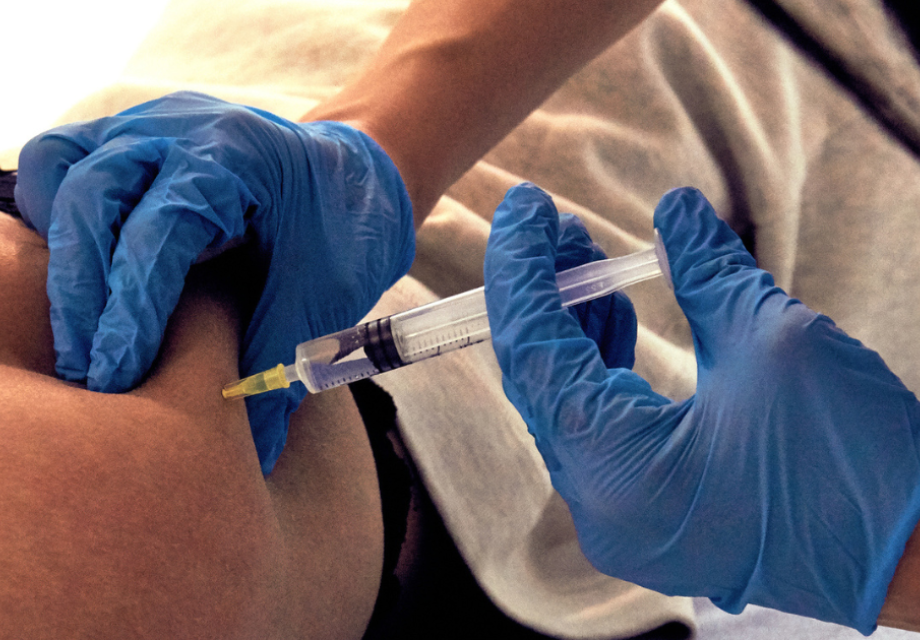
People only need to get the CAB-LA injection every two months instead of taking a PrEP pill every day. The injection comes as two shots, which are injected into the buttocks.
A vaginal ring can be used for 28 days before it needs to be replaced. The ring contains antiretroviral drugs, which are released into the vagina to prevent HIV infection. It is made out of a flexible silicon and looks similar to a contraceptive ring.
Why is this important?
Last year, 54,000 people in Uganda got HIV. More than two-thirds were young people (ages 15-24), with young women most at risk. Although HIV infections are generally declining in the country, they are not falling as quickly as is needed to end the AIDS epidemic by 2030. Offering young women and others at heightened risk of HIV different ways to protect themselves is an essential part of controlling the epidemic.
What is happening?
Rolling out any new HIV medication or product goes through these four stages:
- Regulatory approval
- National policies and clinical guidelines released
- Pilot implementation studies
- General rollout
Uganda has now approved the vaginal PrEP ring for use. And if it approves CAB-LA it will be only the third African country to do so, following Zimbabwe and Malawi.
National PrEP policies and guidelines have been updated to include both products. This means the country is now entering the third stage of the roll-out process for the vaginal ring: pilot implementation studies. Implementation pilots for CAB-LA will follow once it has been approved.
Uganda’s Joint Medical Research Centre has confirmed that adolescents and young people aged 12 to 24 will be asked to take part in clinical trials for CAB-LA and will be followed for 24 months.
What does this mean for HIV services?
Although it will take time for both PrEP products to become widely available in Uganda, these latest developments suggest things are moving forward.
But the cost of CAB-LA remains an issue, and this might limit how widely the injection is available in Uganda. The drug manufacturer of CAB-LA is currently in negotiations to lower the cost of CAB-LA in low- and middle-income countries and to let other manufactures make the drug. But whatever is negotiated, the cost of providing injections is likely to be far higher than the cost of providing PrEP pills.
There will also be logistical issues to deal with, as CAB-LA needs to be stored in refrigerators.
Despite these challenges, the Ugandan government looks to be committed to offering more choice when it comes to HIV prevention.
For women, having access to PrEP products is particularly important because women can be more in control of using PrEP than condoms. PrEP pills have not been as popular as expected in Uganda and other countries in the region. But providing PrEP in products that are more discreet to use and do not have to be taken every day might improve uptake.
Discussing the vaginal ring, Dr Vincent Bagambe, the director for planning and strategic information at the Uganda Aids Commission, said: “This option will put HIV prevention in the hands of women. If all women vulnerable to HIV can embrace it, it will be a game changer.”
This article was amended on 17 May 2023 to correct an earlier version which stated that CAB-LA had already been approved for use in Uganda.
Get our news and blogs by email
Keep up-to-date with all our latest news stories and blogs by signing up to the Be in the KNOW news digest.